Photoinduced and electron-induced chemical reactions
Chemical reactions initiated by light or by collisions with electrons play an important role in atmospheric chemistry, astrochemistry, synthetic chemistry, and biology, amongst other areas. Understanding the mechanisms of these reactions in detail offers new insight into a range of vital physical and chemical processes, ranging from the breaking of a single chemical bond all the way through to complex multistep processes occurring in biological systems.
We study photoinduced and electron-induced chemistry in the gas phase, using velocity-map imaging to record scattering distributions of reaction products and covariance-map imaging to investigate correlations between different products. You can see an animation of a velocity-map imaging experiment here. We analyse the scattering distributions to learn about the forces and energetics that drive the chemical reaction under study, gaining considerable insight into the physics underlying chemical reactivity.
Velocity-map imaging has been used for some time to study the photochemistry of small molecules, and we have put considerable effort into developing the capability to study larger chemical systems of interest to the broader chemical community. Larger molecules have many more fragmentation pathways than smaller molecules, requiring the development of multimass imaging techniques. Key requirements are a universal ionization technique (we use either a VUV 118 nm laser source or an electron beam) and an ultrafast imaging detector capable of recording multiple images on the microsecond timescale of a time-of-flight mass spectrum (see Pixel Imaging Mass Spectrometry below).
In the past we have studied N,N-dimethylformamide (DMF), a model for peptide bond fragmentation, as well as various molecules of interest in organic photochemistry. We are currently studying photon and electron-induced chemistry of a range of model biomolecules relevant to radiation damage to DNA, and formation and destruction of polycyclic aromatic hydrocarbons (PAHs), which are important molecular species within interstellar gas clouds. As an example, a multimass velocity-map imaging data set recorded for DMF using the PImMS ultrafast imaging sensor is shown below.
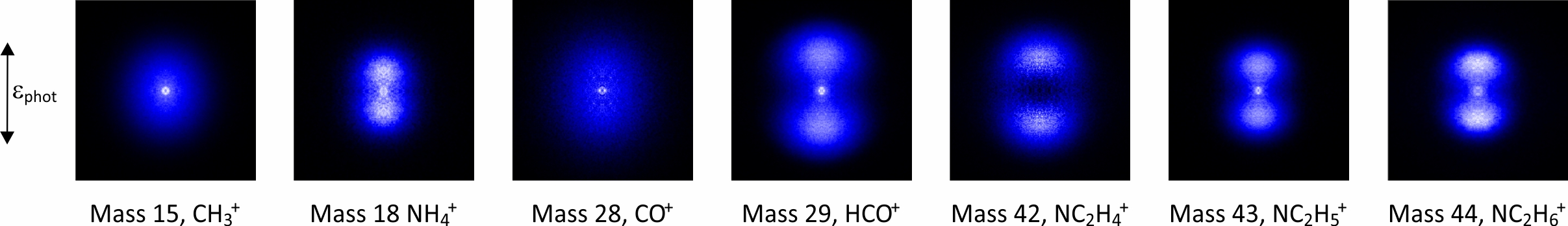
|
Ultra-fast detectors for time-of-flight imaging
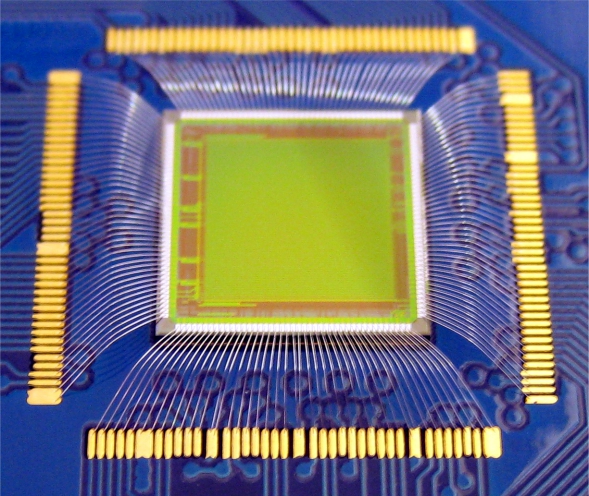
|
We are part of the PImMS (Pixel Imaging Mass Spectrometry) consortium, a group of researchers who are working to develop ultra-fast imaging sensors suitable for applications in time-of-flight imaging. For example, the sensors allow velocity-map or spatial-map images to be acquired for each mass peak in a time-of-flight mass spectrum, opening up a range of new applications in mass spectrometry. The PImMS sensors are being used by several research groups within Oxford, Harwell, and Bristol, and have travelled around the world for experiments in Brookhaven, Ottawa, Hamburg, Aarhus, and numerous other locations. |
Spectroscopic measurements in surgical decision making

|
We are working with clinicians and researchers in cardiology, vascular surgery, and neurosurgery at the John Radcliffe hospital to develop various types of spectroscopy and mass spectrometry for the analysis of clinical samples. In the past we have worked on genetic subtyping of gliomas, a type of brain tumour, by Raman spectroscopy. A second project focused on the use of visible and near-infrared reflectance spectroscopy and spectral imaging in combination with machine learning to characterise thrombus removed from the coronary arteries of STEMI (heart attack) patients and to correlate these measurements with prognostic outcomes for these patients. Current projects use atmospheric solids analysis probe mass spectrometry (ASAP-MS) to characterise plasma from STEMI and abdominal aortic aneurysm patients with the goal of answering a number of clinical questions relating to patient prognosis. We are also using ASAP-MS to investigate the development of a rapid tissue typing methodology for applications in neuropathology. |
Optical microcavities for chemical sensing
Over the past few years we have been working with Prof. Jason Smith's group in Oxford Materials to develop miniature optical cavities for applications in solution-phase chemical sensing and nanoparticle characterisation. Microcavities are only a few wavelengths in length, giving them interesting optical properties, and contain tiny quantities of liquid, often only a few tens of femtolitres. As with any optical cavity, light forms standing waves known as cavity modes at well-defined frequencies within the cavities. By tracking changes in the frequencies and intensities of individual cavity modes when a sample is introduced into the cavity, we can detect and characterise single nanoparticles, and perform chemical sensing down to the few-molecule level. We are in the process of commercialising this work for applications in environmental sensing via a new spin-out company.
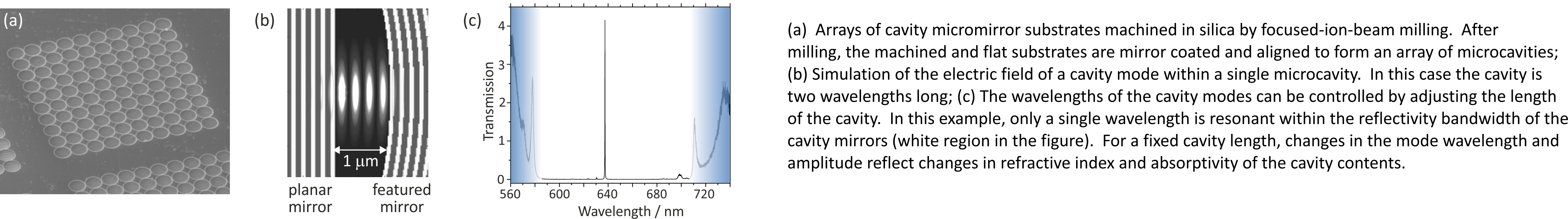
|